- Research
- Open access
- Published:
Utilization of EHRs for clinical trials: a systematic review
BMC Medical Research Methodology volume 24, Article number: 70 (2024)
Abstract
Background and objective
Clinical trials are of high importance for medical progress. This study conducted a systematic review to identify the applications of EHRs in supporting and enhancing clinical trials.
Materials and methods
A systematic search of PubMed was conducted on 12/3/2023 to identify relevant studies on the use of EHRs in clinical trials. Studies were included if they (1) were full-text journal articles, (2) were written in English, (3) examined applications of EHR data to support clinical trial processes (e.g. recruitment, screening, data collection). A standardized form was used by two reviewers to extract data on: study design, EHR-enabled process(es), related outcomes, and limitations.
Results
Following full-text review, 19 studies met the predefined eligibility criteria and were included. Overall, included studies consistently demonstrated that EHR data integration improves clinical trial feasibility and efficiency in recruitment, screening, data collection, and trial design.
Conclusions
According to the results of the present study, the use of Electronic Health Records in conducting clinical trials is very helpful. Therefore, it is better for researchers to use EHR in their studies for easy access to more accurate and comprehensive data. EHRs collects all individual data, including demographic, clinical, diagnostic, and therapeutic data. Moreover, all data is available seamlessly in EHR. In future studies, it is better to consider the cost-effectiveness of using EHR in clinical trials.
Introduction
Clinical trials are of high importance for medical progress [1]. Well designed and well-executed clinical trial studies provide the foundational data for evidence-based medicine [2], which are the standard for evaluating the benefits and harms of medical interventions [3]. Numerous factors lead to the success of clinical trials, such as appropriate trial design(e.g. randomization, blinding, and controls), thorough training of research staff, and recruitment of an adequate sample size by identifying and enrolling qualified participants in a timely manner [4, 5] and maintaining good participation through study completion [2, 6].
Strategic selection of study sites with access to suitable patient populations can optimize recruitment. Moreover, developing practical yet scientifically sound protocols through careful planning and analysis helps ensure trials are completed in an accurate and cost-effective manner [2]. Traditionally, many trials have relied heavily on physician referrals to identify and attract potential participants [7]. While essential, sole dependence on this approach has limitations including referral bias and logistical challenges that could hamper recruitment. To strengthen the recruiting process, manually reviewing patient’s electronic records to identify and diagnose eligible candidates for clinical trials has become a standard practice [8]. However, this manual chart review method is often time-consuming and resource-intensive [9].
To modernize clinical recruitment and conduct, new tools have been developed that enable data-driven insights into patient populations within EHR systems [10]. In fact, to digitalize processes, the TransCelerate e-Resource initiative, launched in January 2016, aims to facilitate understanding the e-resource landscape and the optimal use of electronic data resources to improve clinical science and clinical trial implementation for stakeholders. The eSource initiative also aligns well with other TransCelerate initiatives designed to help modernize trial execution and ways to enroll patients in clinical trials [38].
EHR systems contain comprehensive demographic, medical and treatment history collected during routine care, which offer potential to efficiently pre-scan, identify and recruit appropriate patients for clinical trials [11,12,13,14]. Specifically, recruiting patients through the EHR allows pre-assessment of eligibility criteria, selection of targeted population, and automated outreach to participants [15]. EHRs also provide ongoing access to detailed patient data that may decrease redundant measurements and data collection during trials [12]. Overall, EHR-enabled recruitment and workflow processes have potential to make clinical trials more cost-effective and feasible [11]. This study conducted a systematic review to identify the applications of EHR in supporting and enhancing clinical trials.
Method
Study design
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Literature search
A systematic search of PubMed was conducted on 12/3/2023 to identify relevant studies on the use of EHRs in clinical trials. The search included a combination of Medical Subject Headings (MeSH terms) and keywords related to electronic health records (EHR OR electronic medical record) AND clinical trials. The search was limited to title and abstract fields. No date or language limits were applied. The specific Boolean search syntax was:
-
("EHR"[Title/Abstract] OR "Electronic health record"[Title/Abstract] OR "Electronic health records"[Title/Abstract] OR "EMR"[Title/Abstract] OR "Electronic medical record"[Title/Abstract] OR "Electronic medical records"[Title/Abstract]) AND (clinical trial* [Title/Abstract]).
Reference lists of included studies were hand-searched to identify additional relevant articles. The search was performed without any time limit.
Eligibility criteria
Studies were included if they (1) were full-text journal articles, (2) were written in English, (3) examined applications of EHR data to support clinical trial processes (e.g. recruitment, screening, data collection). Reviews, letters, abstracts, editorials and other non-research studies were excluded.
Study selection and data extraction
Two researchers (EM and LRK) independently screened titles and abstracts of retrieved records to identify potentially eligible studies. After obtaining full texts of potential articles, the two investigators independently assessed eligibility based on predefined criteria. Disagreements were resolved through discussion and consensus. A form was used by two reviewers to extract data on: study design, EHR-enabled process(es), related outcomes, and limitations.
Evidence synthesis
A qualitative synthesis was conducted summarizing key outcomes and limitations of included studies grouped by the EHR-enabled process examined. The study authors met regularly to discuss consensus on findings.
Result
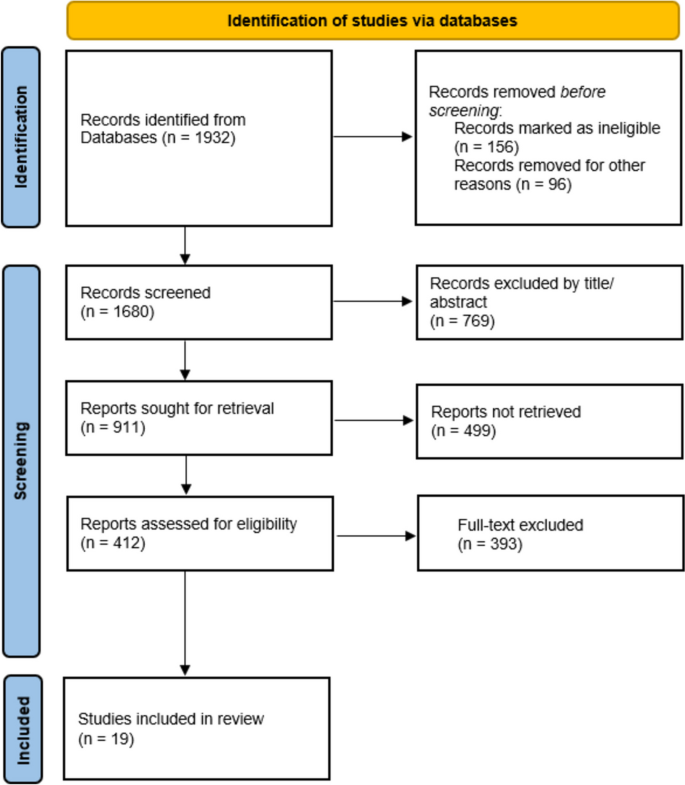
The systematic literature search yielded 2161 records, out of which 312 were selected for full-text review after screening titles and abstracts. After conducting a thorough review of the full-texts and resolving disagreements regarding 2 articles, a total of 19 studies that met the predefined eligibility criteria were included in the final qualitative synthesis (Fig. 1).
Characteristics of included studies
The key characteristics of the 19 included studies are summarized in Table 1. The studies were published in a variety of international journals, with the majority (14/19) from the United States. The remaining studies originated from China, Switzerland, Germany, Belgium, and Finland. The sample sizes ranged from 165 to 5,529,407 patients.
Clinical Trial Processes and Outcomes
Nineteen studies examined the impacts of EHR use on clinical trial processes and outcomes. Table 2 summarizes the key findings on EHR applications for recruitment, screening, data collection, and trial design. Overall, the included studies consistently demonstrated that utilization of EHR data improved clinical trial feasibility and efficiency in the following ways:
-
Recruitment: 19 studies evaluating EHR-enabled recruitment have reported increased enrollment efficiency compared to standard practices.
-
Screening: In 5 studies, EHR pre-screening excluded patients prior to full eligibility screening, reducing unnecessary procedures.
-
Data collection: In 3 studies using EHR data reduced data collection costs compared to standard methods.
-
Trial Design: In one study examining this application, EHR data informed optimization of eligibility criteria to improve statistical power for a COVID-19 trial.
Purposes of using EHR
The most frequent application of EHR data was to identify and recruit eligible participants into clinical trials. By containing diverse information on demographics, clinical history, diagnoses, and more, EHRs allowed pre-screening and outreach to potential candidates that met enrollment criteria. In several studies, EHR data was leveraged for secondary research purposes including data collection, data analysis and optimizing trial design [16, 27,28,29,30, 38]. Specifically, one study utilized EHR data from COVID-19 patients to inform eligibility criteria selection and improve statistical power for COVID-19 trials [23]. Overall, the primary use case was to enable secondary research applications of EHR data beyond routine clinical care to facilitate clinical trial processes. Key limitations of these applications included potential for selection bias, generalizability concerns in single health system populations, and heterogeneity in methods and endpoints assessed across studies. Further investigation using standardized methodology is needed to realize the full potential of EHR-enabled clinical research.
Discussion
This systematic review aimed to identify applications and impacts of electronic health record (EHR) use in clinical trials. The included studies demonstrated EHR data has been leveraged to serve various key functions, including identifying eligible participants, facilitating recruitment, enabling data collection and analysis, and optimizing trial design.
In one study, EHR data was from 59639 patients who encountered health care system. The results showed that the EHR data could be used as a promising clinical tool to assist physicians in early identification of patients suitable for palliative care counseling [35]. Although this study used EHR for therapeutic purposes, it can be concluded that EHR data is very effective in identifying individuals with any target.
Another study found that primary care electronic health record data could be used effectively to identify patients who have been prescribed specific medications and patients who are potentially experiencing drug side effects [36]. In general, based on the results of this study, EHR can also be utilized in clinical trials for purposes other than patient care and in particular for the secondary use of this tool. In fact, according to the studies [20, 28,29,30,31,32], the use of EHR serves various purposes in clinical trials, including identifying eligible participants, facilitating their recruitment and analyzing patient data to assess outcomes and measure the safety and efficacy of the intervention.
EHRs can be used as a database for the use of data needed in clinical trials. For example, a study in Brazil used EHR data to obtain benchmark for stroke patients [37].
According to the results of the present study, the use of EHR in conducting clinical trials is very helpful. Therefore, it is better for researchers to use EHR in their studies for easy access to more accurate and comprehensive data. EHRs collects all individual data, including demographic, clinical, diagnostic, and therapeutic data. So that all data is available seamlessly. Real-time access to patient data directly from EHRs could eliminate the need for manual data entry, minimizing errors and ensuring data integrity.
Moreover, EHRs enable the seamless integration of clinical trial data with other relevant health information, providing a more comprehensive picture of patient health and facilitating the evaluation of long term outcomes. In future studies, it is better to consider the cost-effectiveness of using EHR in clinical trials. Because due to the increasing use and effectiveness of using EHR in clinical trials, its cost-effectiveness should also be determined. Also, conducting such research would be useful for the wider scientific community. Also, in future studies, many metrics can be investigated and reported to reflect the effectiveness of EHR for patient registration. Also, some statistics can be shown to illustrate this.
One of the limitations of the present study was the lack of access to some databases due to sanctions. Another limitation is the lack of a similar study that comprehensively examines the role and effectiveness of EHR in clinical trials. There are also a small number of studies that have examined the effectiveness, how the EHR is used, and its uses in clinical trials.
Another limitation is related to the comparison of the studies included in this study, considering that the EHR system used in different countries, even in each country, is very different in many aspects, including the type of system used, the culture of each country, the level of EHR implementation, technical infrastructure, etc. Therefore, the comparison between systems was one of the limitations of this study.
Conclusions
According to the results of the present study, it can be concluded that EHR in clinical trials is used for various purposes. While promising, several limitations should be considered when interpreting the evidence. Many EHRs may rely on single health system populations, limiting generalizability of findings. Heterogeneity in methods and endpoints used to evaluate the same EHR processes is another issue to be considered. Additional limitations included potential for selection and referral bias. More research is needed to develop standardized methodology and reporting for EHR-enabled clinical trials. Future directions of the research should be to optimize EHRs for supporting clinical trials. This may be realized through enhanced interoperability and data sharing between EHR systems to facilitate multi-site and diverse patient populations trials and expand access to diverse patient populations beyond single health systems. Standardization of data formats, development of shared platforms, and policies enabling access are needed. Integration of clinical trial-specific modules into EHRs is required to simplify participant screening, recruitment, enrollment, and data collection. This could include dashboards, automated alerts, and documentation templates. Advanced analytics and machine learning applied to EHR data can also be a part of agenda for future research. Stronger privacy protections and cybersecurity measures should be in place to securely operationalize EHR data for research while maintaining patient confidentiality.
There is also gap in cost-effectiveness studies to quantify financial benefits and guide investments in EHR-enabled research infrastructure.
Availability of data and materials
Not applicable.
References
Kohl CD, Garde S, Knaup P. Facilitating secondary use of medical data by using openEHR archetypes. Stud Health Technol Inform. 2010;160(Pt 2):1117–21.
Laaksonen N, Varjonen J-M, Blomster M, Palomäki A, Vasankari T, Airaksinen J, et al. Assessing an electronic health record research platform for identification of clinical trial participants. Contemp Clin Trials Commun. 2021;21:100692.
Bothwell L, Greene J, Podolsky S, Jones D. Assessing the gold standard-lessons from the history of RCTs. N Engl J Med. 2016;374(22):2175–81.
Foster JM, Sawyer SM, Smith L, Reddel HK, Usherwood T. Barriers and facilitators to patient recruitment to a cluster randomized controlled trial in primary care: lessons for future trials. BMC Med Res Methodol. 2015;15(1):1–9.
Farrell B, Kenyon S, Shakur H. Managing clinical trials. Trials. 2010;11(1):1–6.
Menachemi N, Collum TH. Benefits and drawbacks of electronic health record systems. Risk Manag Healthcare Policy. 2011;4:47.
Mapstone J, Elbourne DD, Roberts IG. Strategies to improve recruitment to research studies. Cochrane Database of Systematic Reviews. 2002(3).
Vickers AJ. How to improve accrual to clinical trials of symptom control 2: design issues. J Soc Integr Oncol. 2007;5(2):61.
Doods J, Bache R, McGilchrist M, Daniel C, Dugas M, Fritz F. Piloting the EHR4CR feasibility platform across Europe. Methods Inf Med. 2014;53(04):264–8.
Kellar E, Bornstein SM, Caban A, Célingant C, Crouthamel M, Johnson C, et al. Optimizing the use of electronic data sources in clinical trials: the landscape, part 1. Ther Innov Reg Sci. 2016;50(6):682–96.
Mc Cord KA, Ewald H, Ladanie A, Briel M, Speich B, Bucher HC, et al. Current use and costs of electronic health records for clinical trial research: a descriptive study. CMAJ Open. 2019;7(1):E23.
Zuidgeest MG, Goetz I, Groenwold RH, Irving E, van Thiel GJ, Grobbee DE, et al. Series: pragmatic trials and real world evidence: paper 1 introduction. J Clin Epidemiol. 2017;88:7–13.
Mc Cord KA, Salman RAS, Treweek S, Gardner H, Strech D, Whiteley W, et al. Routinely collected data for randomized trials: promises, barriers, and implications. Trials. 2018;19(1):1–9.
Beaver JA, Howie LJ, Pelosof L, Kim T, Liu J, Goldberg KB, et al. A 25-year experience of US food and drug administration accelerated approval of malignant hematology and oncology drugs and biologics: a review. JAMA Oncol. 2018;4(6):849–56.
Li G, Sajobi TT, Menon BK, Korngut L, Lowerison M, James M, et al. Registry-based randomized controlled trials-what are the advantages, challenges, and areas for future research? J Clin Epidemiol. 2016;80:16–24.
Ateya MB, Delaney BC, Speedie SM. The value of structured data elements from electronic health records for identifying subjects for primary care clinical trials. BMC Med Inform Decis Mak. 2016;16:1.
Beresniak A, Schmidt A, Proeve J, Bolanos E, Patel N, Ammour N, et al. Cost-benefit assessment of using electronic health records data for clinical research versus current practices: Contribution of the Electronic Health Records for Clinical Research (EHR4CR) European Project. Contemp Clin Trials. 2016;46:85–91.
Bruland P, McGilchrist M, Zapletal E, Acosta D, Proeve J, Askin S, et al. Common data elements for secondary use of electronic health record data for clinical trial execution and serious adverse event reporting. BMC Med Res Methodol. 2016;16(1):159.
Carrion J. Improving the patient-clinician interface of clinical trials through health informatics technologies. J Med Syst. 2018;42(7):120.
De Moor G, Sundgren M, Kalra D, Schmidt A, Dugas M, Claerhout B, et al. Using electronic health records for clinical research: the case of the EHR4CR project. J Biomed Inform. 2015;53:162–73.
Embi PJ, Jain A, Clark J, Bizjack S, Hornung R, Harris CM. Effect of a clinical trial alert system on physician participation in trial recruitment. Arch Intern Med. 2005;165(19):2272–7.
Ernecoff NC, Wessell KL, Gabriel S, Carey TS, Hanson LC. A novel screening method to identify late-stage dementia patients for palliative care research and practice. J Pain Symptom Manage. 2018;55(4):1152–8.e1.
Kim JH, Ta CN, Liu C, Sung C, Butler AM, Stewart LA, et al. Towards clinical data-driven eligibility criteria optimization for interventional COVID-19 clinical trials. J American Med Inform Assoc. 2021;28(1):14–22.
Kirshner J, Cohn K, Dunder S, Donahue K, Richey M, Larson P, et al. Automated electronic health record-based tool for identification of patients with metastatic disease to facilitate clinical trial patient ascertainment. JCO Clin Cancer Inform. 2021;5:719–27.
Laaksonen N, Varjonen JM, Blomster M, Palomäki A, Vasankari T, Airaksinen J, et al. Assessing an electronic health record research platform for identification of clinical trial participants. Contemp Clin Trials Commun. 2021;21:100692.
Li M, Cai H, Nan S, Li J, Lu X, Duan H. A Patient-screening tool for clinical research based on electronic health records using openEHR: development study. JMIR Med Inform. 2021;9(10):e33192.
Meystre SM, Heider PM, Kim Y, Aruch DB, Britten CD. Automatic trial eligibility surveillance based on unstructured clinical data. Int J Med Informatics. 2019;129:13–9.
Miotto R, Weng C. Case-based reasoning using electronic health records efficiently identifies eligible patients for clinical trials. J American Med Inform Assoc. 2015;22(e1):e141–50.
Nelson SJ, Drury B, Hood D, Harper J, Bernard T, Weng C, et al. EHR-based cohort assessment for multicenter RCTs: a fast and flexible model for identifying potential study sites. Journal of the American Medical Informatics Association : JAMIA. 2021.
Ni Y, Bermudez M, Kennebeck S, Liddy-Hicks S, Dexheimer J. A real-time automated patient screening system for clinical trials eligibility in an emergency department: design and evaluation. JMIR Med Inform. 2019;7(3):e14185.
O’Brien EC, Raman SR, Ellis A, Hammill BG, Berdan LG, Rorick T, et al. The use of electronic health records for recruitment in clinical trials: a mixed methods analysis of the harmony outcomes electronic health record ancillary study. Trials. 2021;22(1):465.
Rogers JR, Liu C, Hripcsak G, Cheung YK, Weng C. Comparison of clinical characteristics between clinical trial participants and nonparticipants using electronic health record data. JAMA Netw Open. 2021;4(4):e214732.
Sun Y, Butler A, Diallo I, Kim JH, Ta C, Rogers JR, et al. A framework for systematic assessment of clinical trial population representativeness using electronic health records data. Appl Clin Inform. 2021;12(4):816–25.
Zimmerman LP, Goel S, Sathar S, Gladfelter CE, Onate A, Kane LL, et al. A novel patient recruitment strategy: patient selection directly from the community through linkage to clinical data. Appl Clin Inform. 2018;9(1):114–21.
Guo A, Foraker R, White P, Chivers C, Courtright K, Moore N. Using electronic health records and claims data to identify high-risk patients likely to benefit from palliative care. American J Managed Care. 2021;27(1):e7–15.
Cole AM, Stephens KA, West I, Keppel GA, Thummel K, Baldwin L-M. Use of electronic health record data from diverse primary care practices to identify and characterize patients’ prescribed common medications. Health Informatics J. 2020;26(1):172–80.
Valêncio RFZ, Souza JTd, Winckler FC, Modolo GP, Ferreira NC, Bazan SGZ, et al. Semi-automated data collection from electronic health records in a stroke unit in Brazil. Arquivos de Neuro-Psiquiatria. 2021.
US Food and Drug Administration. Guidance for industry: electronic source data in clinical investigations. Silver Spring MD. 2013;16:15.
Acknowledgements
The research protocol was approved & Supported by Student Research Committee, Tabriz University of Medical Sciences (grant number: IR.TBZMED.VCR.REC.1401.152).
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
E.M. Writing the main manuscript text, Data curation, prepared figures, writing – review & editing. L.K. Validation, Investigation, Conceptualization, Methodology, Supervision, All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study is approved by ethical committee of Tabriz University of Medical Sciences (IR.TBZMED.VCR.REC.1401.152).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kalankesh, L.R., Monaghesh, E. Utilization of EHRs for clinical trials: a systematic review. BMC Med Res Methodol 24, 70 (2024). https://doi.org/10.1186/s12874-024-02177-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12874-024-02177-7